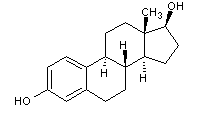
Structural formula
| Business number | 013E |
|---|---|
| Molecular formula | C18H24O2 |
| Molecular weight | 272.38 |
| label |
Ovocylin, Dihydroxyestrin, Estr-1,3,5-(10)-triene-3β,17β-diol, 1,3,5-Estratriene-3,17β-diol, 17β-Estradiol, 3,17β-Dihydroxy-1,3,5(10)-estratriene, Dihydrofolliculin, Estradiol; Estradiol; Courtship diol; Dihydroxyestradiol; Estradiol, Enzymes·Proteins·Peptides |
Numbering system
CAS number:50-28-2
MDL number:MFCD00003693
EINECS number:200-023-8
RTECS number:KG2975000
BRN number:1914275
PubChem number:24278426
Physical property data
1. Properties: White or milky white leaf-like or needle-like crystals (ethanol solution); odorless. Stable in air
2. Density (g/mL, 25/4℃): Not determined
3. Relative vapor density (g/mL, air=1): Not determined Determined
4. Melting point (ºC): 178-179 °C (lit.)
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, 5.2 kPa): Not determined
7. Refractive index: 80.4 ° (C=1, Dioxane)
8. Flash point (ºC): Not determined
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25 ºC): Undetermined
12. Saturated vapor pressure (kPa, 60 ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Oil and water (octanol/water) distribution Log value of coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V): Undetermined 19. Solubility : Easily soluble in ethanol, soluble in acetone, chloroform, dioxane and alkali solution, slightly soluble in vegetable oil, almost insoluble in water
Toxicological data
Acute toxicity:
Main irritant effects:
On skin: Irritation to skin and mucous membranes
On eyes: Irritating effects.
Sensitization: No known sensitizing effects.
Ecological data
General notes
Water hazard level 3 (Germany�Example) (Self-assessment via list) This substance is extremely hazardous to water.
Do not allow this product to come into contact with groundwater, waterways or sewage systems, even in small amounts.
Even extremely small amounts of product seeping into the ground can pose a hazard to drinking water
Do not discharge materials into the surrounding environment without government permission.
Molecular structure data
1. Molar refractive index: 79.50
2. Molar volume (cm3/mol): 232.6
3. Isotonic specific volume (90.2K ): 615.4
4. Surface tension (dyne/cm): 48.9
5. Polarizability (10-24cm3): 31.51
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: 9
6. Topological molecule polar surface area 40.5
7. Number of heavy atoms: 20
8. Surface charge: 0
9. Complexity: 382
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 5
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
The effect of estrogen can promote the development of secondary sexual characteristics and the final formation of sexual organs in underage girls. In adult women, in addition to maintaining the above-mentioned secondary sexual characteristics, it can also cause a series of changes in the endometrium to produce cyclic menstruation. It can also increase the activity of the uterus and fallopian tubes and increase the sensitivity of uterine muscles to oxytocin. In addition, estrogen can also increase the deposition of bone calcium salts and accelerate epiphyseal closure. In large doses, it can increase serum triglycerides and phospholipids, causing water and sodium retention. At larger doses, estrogen can also act on the hypothalamic-pituitary system, inhibit the secretion of gonadotropins and prolactin, and offset the main effects of androgens.
Storage method
This product should be sealed and stored in a dry place away from light.
Synthesis method
1. Use androst-4-en-19-desmethyl-3,17-dione to introduce bromine at the 2- and 6-positions through bromination, and then add bromine to 4-ethyl-2-methylpyridine In the presence of dehydrobromination (elimination), 2,4,6-androstatriene-19-desmethyl-3,17-dione is generated, which is then cracked at high temperature to generate estrone, and then lithium aluminum hydrogen is reduced to produce Get this product.
Purpose
1. Intermediates of estradiol valerate and estradiol benzoate.
2.Estrogen drugs. It is used for functional uterine bleeding, primary amenorrhea, menopausal syndrome and prostate cancer.
extended-reading:https://www.newtopchem.com/archives/45114extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/Dimethylaminoethoxyethanol-CAS-1704-62-7-N-dimethylethylaminoglycol.pdfextended-reading:https://www.bdmaee.net/monobutyltin-oxide-2/extended-reading:https://www.morpholine.org/high-quality-cas-108-01-0-nn-dimethyl-ethanolamine-2-dimethylamineethanol-dmea-dimethylethanolamine/extended-reading:https://www.newtopchem.com/archives/827extended-reading:https://www.bdmaee.net/wp-content/uploads/2021/05/2-10.jpgextended-reading:https://www.newtopchem.com/archives/category/products/page/9extended-reading:https://www.newtopchem.com/archives/1820extended-reading:https://www.cyclohexylamine.net/cyclohexylamine/extended-reading:https://www.newtopchem.com/archives/185


