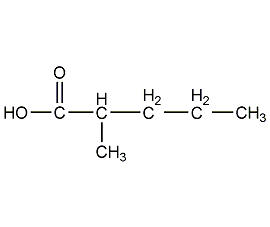
Structural formula
| Business number | 02CE |
|---|---|
| Molecular formula | C6H12O2 |
| Molecular weight | 116.16 |
| label |
a-methylpentanoic acid, Methylpropyl acetate, 2-Methylpentanoic acid, a-Methylvaleric acid, Methylpropylacetic acid, acidic solvent |
Numbering system
CAS number:97-61-0
MDL number:MFCD00002671
EINECS number:202-594-9
RTECS number:YV7700000
BRN number:1720655
PubChem number:24901311
Physical property data
- Characteristics: It solidifies like ice at room temperature, and becomes a colorless liquid above room temperature. Strong pungent spicy smell.
- Density (g/mL, 20℃): 0.931
- Relative density (20℃, 4℃): 0.9230
- Melting point (ºC): -85
- Boiling point (ºC, normal pressure): 195.6~197
- Refractive index at normal temperature (n20): 1.4136
- Refractive index (n20D): 1.414
- Flash point (ºC): 91
- Ratio Optical rotation (º): Not determined
- Autoignition point or ignition temperature (ºC): 378
- Vapor pressure (mmHg,ºC): Not determined
- Saturated vapor pressure (kPa, ºC): Undetermined
- Heat of combustion (KJ/mol): Undetermined
- Critical temperature (ºC): Undetermined
- Critical pressure (KPa): Undetermined
- Log value of oil-water (octanol/water) partition coefficient: Undetermined
- Explosion upper limit (%, V/V): 63
- li>
- Lower explosion limit (%, V/V): 1.3
- Solubility: soluble in water and ethanol, miscible with some essential oils.
Toxicological data
1. Skin/eye irritation: Start irritation test: rabbit skin contact, 500mgREACTION SEVERITY, mild reaction; Standard Dresser test: rabbit skin contact, 500mg/24HREACTION SEVERITY, moderate reaction; 2. Acute toxicity: Rat oral LD50 :2040mg/kg; Rabbit skin contact LD50: 2500mg/kg;
Ecological data
This substance is slightly hazardous to water.
Molecular structure data
1. Molar refractive index: 31.36
2. Molar volume (cm3/mol): 122.5
3. Isotonic specific volume (90.2K ): 290.0
4. Surface tension (dyne/cm): 31.3
5. Polarizability (10-24cm3): 12.43
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 3
5. Number of tautomers: none
6. Topological molecule polar surface area 37.3
7. Number of heavy atoms: 8
8. Surface charge: 0
9. Complexity: 78.6
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 1
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Avoid contact with oxides and alkali.
2. Exist in flue-cured tobacco leaves, oriental tobacco leaves, and mainstream smoke.
3. Naturally found in guava, pepper, and black tea.
Storage method
Store in a cool, ventilated warehouse. Keep away from fire and heat sources. Protect from direct sunlight. The packaging is sealed. They should be stored separately from oxidants and strong alkali, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. Suitable materials should be available in the storage area to contain spills.
Synthesis method
1. Tobacco: OR, 44, 49; FC, 40; prepared by catalytic hydrogenation of 2-methylvaleraldehyde or by reaction of 2-chloropentane with sodium and carbon dioxide under pressure.
Purpose
1. Used in baked goods, frozen dairy products, and puddings.
extended-reading:https://www.bdmaee.net/dabco-ne600-catalyst-cas10861-07-1-evonik-germany/extended-reading:https://www.bdmaee.net/nt-cat-pc5-catalyst-cas3030-47-5-newtopchem/extended-reading:https://www.bdmaee.net/dibutyltin-oxide/extended-reading:https://www.cyclohexylamine.net/category/product/page/22/extended-reading:https://www.newtopchem.com/archives/45053extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/16.jpgextended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/-BX405-low-odor-amine-catalyst-BX405–BX405-polyurethane-catalyst.pdfextended-reading:https://www.newtopchem.com/archives/929extended-reading:https://www.bdmaee.net/anhydrous-tin-chloride/extended-reading:https://www.newtopchem.com/archives/45145


